Internal Research -- Preclinical Evaluations
Memory is one of the diverse cognitive abilities humans possess that can be studied validly in animals. DNS's scientists have been at the forefront of developing models of memory in normal and genetically modified animals. Such models have helped identify effective compounds for human clinical testing.
As proof that augmenters of the CREB pathway are legitimate memory enhancers, DNS scientists modified contextual learning protocol to produce four-day memory retention in normal mice (C57/Bl6) near zero after one training trial, while five training trials yielded maximum levels of memory retention. In this manner, the amount of long-term memory formation could be quantified after "strong" (5 trials) versus "weak" (2 trials) training, thereby effectively assaying the primary behavioral property associated with modulation of CREB (augmenting the CREB pathway will reduce the amount of practice required to induce long-term memory formation). Under these conditions, HT-0712 enhanced memory formation after weak training without affecting memory after strong training (Figure 8).

Figure 8. HT-0712 enhances memory in normal, young mice.
With a modified contextual learning task (See Text), five training trials (strong) produces maximal long-term memory, while two training trials (weak) is submaximal. When a single dose of HT-0712 is delivered intraperitoneally either 20 minutes before training (not shown) or up to 60 minutes after training (shown), weak training is sufficient to produce maximal long-term memory (four-day retention). In contrast, HT-0712 has no effect on the maximal memory usually produced by strong training. Thus, the "CREB signature" is to enhance memory by reducing the amount of training required to produce long-term memory.
One generally might not expect that a "memory enhancer" would have no effect on maximal memory produced by strong training. This result is similar to that originally produced in fruit flies via genetic manipulations, however, suggesting this "CREB signature" is a fundamental behavioral property of CREB-dependent long-term memory formation. The relation between CREB function and memory is subtle and specific; maximal memory is produced from submaximal amounts of training when CREB function is augmented. These results constitute an important proof-of-principle: Drugs that enhance memory can be identified rationally from assays of CREB biochemistry.
Facilitation of age-associated memory impairment (AAMI) in mice
Having established that augmenters of the CREB pathway can act as memory enhancers in normal animals, a more clinically relevant goal was to assess HT-0712's ability to facilitate memory formation in various animals' models of cognitive dysfunction. To assess AAMI in mice, the contextual learning task described above was modified. Mice were taught to associate a tone (sound) with a reinforcement. When tone and reinforcement are present together, young (3 months) and old (18 months) mice both can remember the tone for several days. When tone ends several seconds before reinforcement occurs (referred to as "trace conditioning"), however, old mice have difficulty remembering the task (Figure 9a). This task is analogous to "remembering a phone number" for a few minutes after looking it up. Such deficits in "working memory" are quite common in older people. HT-0712 facilitates this form of AAMI in mice, rendering old animals with long-term memory similar to that of young mice (Figure 9b).
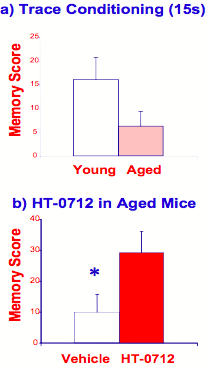
Figure 9. HT-0712 facilitates age-associated memory impairment (AAMI) in mice.
(a) When a tone (sound) stimulus is separated by 15 seconds from reinforcement (trace conditioning), aged mice have more difficulty learning the association than young mice. (b) A single dose of HT-0712 delivered intraperitoneally 20 minutes before training, however, significantly facilitates this AAMI, yielding one-day memory equivalent to that for normal young mice.
Stroke rehabilitation in rats. Rehabilitation after stroke is another clinical context in which memory formation is required to relearn (motor or cognitive) skills damaged by the stroke. Dead cells in the brain cannot be recovered, but the remaining circuitry surrounding the damaged brain region can be "retrained" to assume some of the lost function. Rats partially paralyzed in one forepaw due to a stroke are forced to use the impaired paw to reach for food pellets. With such rehabilitation, motor control of the impaired paw can be restored effectively. Recovery of motor function (reaching) from such exercise-dependent rehabilitation was faster and greater in animals given HT-0712 twenty minutes before training (Figure 10a). HT-0712 also produced a greater return of neural activity in response to reaching for a food pellet in the region of the motor cortex surrounding the damaged brain region (Figure 10b).
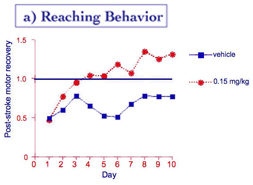
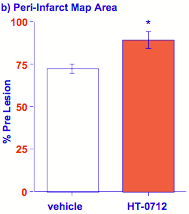
Figure 10. Facilitation of rehabilitation after stroke.
(a) reaching performance (% success) after stroke improves with practice (day) in rats administered vehicle alone. A single injection of HT-0712 (0.15 mg/kg) 20 min before each rehabilitory training session produced a greater recovery level in fewer training sessions. (b) HT-0712 also produced a significant recovery of neural activity over a larger area of cortical tissue surrounding the ischemic lesion than vehicle alone.
Amelioration of cognitive dysfunction in a mouse model of neurodevelopmental disorders
In 1995, when the seminal Drosophila paper on CREB-dependent memory enhancement was published, a report was also published on the identification of a genetic defect associated with Rubinstein-Taybi syndrome (RTS) - an inherited neurodevelopmental disorder that occurs in 1/125,000 births. RTS patients carry defects in the CREB Binding Protein (CBP) gene, which normally binds with activated CREB to regulate gene expression. This molecular discovery presented the obvious hypothesis that the neurodevelopmental disorder of RTS patients might result from biochemical defects in memory formation rather than from any developmental disorders. In fact, this realization was an original motive to form DNS and to search for drugs that up-regulated CREB function. All RTS patients are carriers of one mutant copy and one normal copy of CBP, suggesting that the "CREB switch" was defective but not completely disrupted and, thus, that augmenting upstream biochemistry might yield enough CREB/CBP function for long-term memory formation.
DNS obtained genetically engineered mice carrying a CBP mutation similar to many RTS patients. Like humans, heterozygous CBP+/- mice displayed defects in bone growth and heart function. Importantly, learning and early memory appeared normal but long-term memory was defective in these mice using an "object recognition" task that relies on the natural curiosity that mice have to explore novel objects in their environment. During training, a mouse is presented with two identical, novel objects. It explores them equally by approaching, sniffing and crawling over them. During testing one day later, the mouse is presented with the training object and a second, novel one. Memory of the training object renders it familiar to the mouse, and it then spends more time exploring the new novel object rather than the familiar one. In this task, CBP+/- mutants also showed normal early memory and defective one-day memory (Figure 11a). In the presence of HT-0712, however, long-term memory formation was normal in CBP+/- mutant mice (Figure 11b).
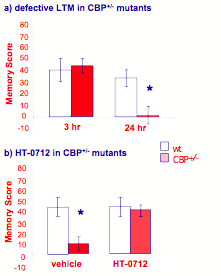
Figure 11. HT-0712 ameliorates a long-term memory defect in mutant CBP+/- mice.
These mutant mice carry the same genetic defect as many people suffering from Rubinstein-Taybi syndrome, an inherited neurodevelopmental disorder. (a) As hypothesized for humans, CBP+/- mutant mice show normal learning and early memory but defective one-day memory in an object recognition task. (b) One injection of HT-0712 twenty min before training eliminates this block of long-term memory formation. More generally, these data suggest that some inherited forms of neurodevelopmental disorders do not result from permanent developmental abnormalities but rather derive from biochemical defects, which can be ameliorated with drug therapy.
More generally, this result anticipates that some inherited forms of neurodevelopmental disorders are not a permanent outcome of altered development but rather may derive from functional defects in the biochemistry of memory formation. The importance of this observation is that drug therapy has overcome a known genetic defect and the study validates the CREB biological pathway for memory formation. Moreover, as the human genome project permits more rapid identification of genes involved in inherited forms of cognitive dysfunction and as basic research continues to elucidate more of the "biochemistry of plasticity," other cases like RTS are becoming apparent. To date, for instance, neurofibromatosis 1, Coffin-Lowry syndrome, Fragile-X syndrome, Angelman's syndrome and Down's syndrome also have been shown to result from defects in genes that are known to participate in the biochemistry of memory formation.

